Complete The Following Acid-base Reaction.
Affiliate one: Acid–Base of operations Reactions
 Equally we will see, organic reactions tin can be classified using a small set of reaction types—the largest and almost all-encompassing of which are those involving acrid–base of operations reactions. Agreement acid–base reactions, therefore, provides a broadly useful conceptual framework inside which to consider a wide range of organic reactions. Although it is likely that y'all have already been introduced to acrid–base of operations reactions (especially if you lot used the Clue general chemical science curriculum[1]), we are going to review this class of reactions in order to emphasize their general features. Our goal is that you learn how to recognize their office in a range of reaction mechanisms; agreement how and why acid–base of operations reactions occur volition give yous to a prepare of tools to sympathise phenomena as diverse as why about drugs are usually administered as in their salt grade (a conjugate acid or base), why biological systems are buffered to specific pH levels (and why different pH levels are establish in different cellular and organismic compartments), and why molecular oxygen (Oii) send systems require a metal ion complex (inside the proteins involved, east.g. myoglobin, hemoglobin, cytochromes). As nosotros will see, acrid–base of operations reactions are by far the most common types of reactions in biological systems.
Equally we will see, organic reactions tin can be classified using a small set of reaction types—the largest and almost all-encompassing of which are those involving acrid–base of operations reactions. Agreement acid–base reactions, therefore, provides a broadly useful conceptual framework inside which to consider a wide range of organic reactions. Although it is likely that y'all have already been introduced to acrid–base of operations reactions (especially if you lot used the Clue general chemical science curriculum[1]), we are going to review this class of reactions in order to emphasize their general features. Our goal is that you learn how to recognize their office in a range of reaction mechanisms; agreement how and why acid–base of operations reactions occur volition give yous to a prepare of tools to sympathise phenomena as diverse as why about drugs are usually administered as in their salt grade (a conjugate acid or base), why biological systems are buffered to specific pH levels (and why different pH levels are establish in different cellular and organismic compartments), and why molecular oxygen (Oii) send systems require a metal ion complex (inside the proteins involved, east.g. myoglobin, hemoglobin, cytochromes). As nosotros will see, acrid–base of operations reactions are by far the most common types of reactions in biological systems.
A quick review of the models of acid–base reactions.
There are a number of ways to talk over acid–base reactions, depending on what aspects of the reaction we desire to highlight. They range from the extremely simplified (and not useful) Arrhenius model, to the Brønsted–Lowry model that we use only for reactions in which protons are transferred, and finally to the Lewis model, which tin can encompass any blazon of acid–base reaction.
Arrhenius: The Arrhenius acid–base model is probably the starting time acid–base model that you were introduced to in the form of your pedagogy. In this model, when an acid dissolves in water it dissociates to release a hydrogen ion (H+); when a base dissolves information technology releases a hydroxide ion (–OH).
Acid: HCl(yard) + H2O [latex]\rightarrow[/latex] H+ (aq) + Cl– (aq) (sometimes written as or HCl(aq))
Base: NaOH(due south) + H2O [latex]\rightarrow[/latex] Na+(aq) + –OH(aq)
Acid–Base Reaction: HCl (aq) + NaOH(aq) [latex]\rightarrow[/latex] NaCl(aq) + H2O (l)[two]
Although unproblematic, the Arrhenius model is non peculiarly useful when it comes to understanding the reactions considered in organic chemical science. This of course raises the obvious question: and then why are we mentioning it? The respond is 2 fold: i) because you might well vaguely recollect it equally a description of acid–base behaviors and ii) so that we can consider why it is not useful and why y'all should not employ it. The Arrhenius acrid–base of operations model applies only when water is the solvent—as we volition come across many organic reactions do not occur in water. The Arrhenius model too, falsely implies that there are free protons (H+) roaming around in water and it restricts bases to those substances that release a hydroxide ion. Finally, it implies that an acid can exist independent of a base of operations—and vice versa, which doesn't make a keen deal of sense.
Brønsted–Lowry: The Brønsted–Lowry model is a much more useful and flexible model for considering acid base reactions. In this model an acid is a proton (H+) donor and a base is a proton acceptor. In the Brønsted–Lowry model you cannot take an acid without a base of operations, and vice versa; the acrid has to donate its H+ to something (the base), and similarly the base has to accept it. The H+ doesn't just "drop off"—information technology is transferred.[3] In the instance of reactions that occur within aqueous solution, the H+ is transferred to a water molecule to form HiiiO+. Consider, equally an example, HCl; in aqueous solution HCl transfer a H+ group to a h2o molecule. The products are H3O+ (the conjugate acid of water) and Cl–, the conjugate base of HCl.
| HCl(g) + | HiiO(50) | ⇆ | H3O+ (aq) | + | Cl–(aq) |
| acid | base of operations | conjugate acid | conjugate base of operations | ||
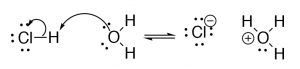
The fundamental point here is that the H+ is transferred from one molecule to the other—it doesn't drop off and and then reattach.
The flexibility of the Brønsted–Lowry model lies in the fact that the base does not necessarily accept to be water. For instance, if we look at the reaction of hydrogen chloride and ammonia (NH3), we run across that the proton transfer from acid to base of operations is analogous to the reaction in water.
| HCl | + | NHiii | ⇄ NH4 + + | Cl– |
| acid | base | conjugate acrid | conjugate base | |
In the Brønsted–Lowry model, as for all chemical reactions considered at the molecular level, there is the possibility for the reaction to opposite, which is denoted by the use of equilibrium arrows (⇄).
At the macroscopic level the extent to which the reaction proceeds (from reactants on the left to products on the right) is adamant by a number of factors. That is, we demand more data to predict (or calculate) the concentrations of reactants and projects at equilibrium. This is information that as well enables us to predict whether the reaction will go along in the forward management (to the right) or not and how the reaction might modify if nosotros add or remove reactants (or products).
We can place a potentially acidic H + because information technology volition exist bonded to a more electronegative atom; the result is that the electron density in the bond will prevarication mainly with the more than electronegative atom (e.grand. O, Northward, or Cl). The upshot is that, for case, an H–O bond will be weakened (require less energy to break); the H will have a big partial positive charge on it, and volition be strongly attracted to basic centers (equally described in the next section). Similarly, simple bases can exist identified by the presence of an cantlet (within the molecule) that has a partial negative accuse; this partial negative accuse arises because the atom (the bones centre) is bonded to less electronegative atoms. At present we add one further consideration, this base center atom also needs to exist able to accept the incoming H+. In practice, this ways that a basic molecule will contain an atom that has a solitary (non-bonding) pair of electrons that can form a bond to the H + .
The Brønsted–Lowry model is useful for acid–base of operations reactions that involve proton transfer, just even so, information technology is express to proton transfer reactions. We also note here that the solvent in which the proton transfer takes place will have an event on the reaction, and we volition return to this idea after in the course. If we extend the Brønsted model to other reactions where a base uses its lone electron pair to course a new bond with an electropositive center, we can expand the class of acid–base reactions even further. Which brings u.s.a. to the adjacent model of acid base of operations chemistry: the Lewis model.
Lewis: The Lewis model allows us to describe exactly the same set of reactions as does the Brønsted–Lowry model, but from a different perspective, and it also allows us to expand on the model. In the Lewis model a base has the ability to donate an electron pair to course a new bond with the acid that accepts this new bond, ofttimes but non always with the concomitant breaking of a bond within the acid molecule. We use same rationale for why the reaction occurs between two oppositely charged centers, just from the perspective of the electrons, rather than the H+. A base must therefore have a lone pair of electrons that tin can accept part in a bond while an acid must have an atom that tin can accept that lone pair of electrons. Using the reaction of HCl and water equally an case, we employ the curved arrow note to denote how the electrons motion between base and acid. Recall[4] that we employ this curved arrow notation to indicate the movement of electron pair
from a source of electrons to a sink. Here the source is the lone pair on the oxygen, and the sink is the hydrogen (which has a δ+ due to its bonding to a Cl) The 2nd arrow moves from the source (the bond betwixt H and Cl, to the sink—the electronegative Cl which ends up with the negative charge, while the O that donated the original electron pair ends up with a positive charge).
The Lewis model encompasses the Brønsted–Lowry model, that is, all Brønsted–Lowry acid–base reactions that tin can be described using the Lewis model. However, the Lewis model extends the range of reaction types that tin exist considered as acid–base reactions. Have for example the reaction of ammonia (NH3) and boron trifluoride (BF3). This reaction is classified as a Lewis acid–base reaction, simply it is non a Brønsted acrid–base of operations reaction.
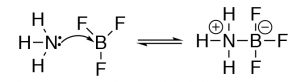
Why apply different models of acid–base of operations chemistry? While at kickoff the thought of using unlike models to explain acid–base of operations chemistry may be a trivial confusing. Why not use the all-encompassing Lewis model for everything? Information technology turns out that both the Brønsted–Lowry and Lewis models are particularly useful depending on the system nether consideration. The trick is to recognize which is the about useful when describing, predicting, and explaining a item type of chemical reaction.[5]
In our explorations in organic chemistry nosotros volition be using both Brønsted–Lowry (proton transfer) and Lewis (electron pair donation) models to describe acid–base chemical science, depending on the type of reaction. In practice, the Brønsted–Lowry model is simple and useful; information technology tells you what is happening (proton transferred from acrid to base) only nada well-nigh the machinery by which the H+ moves. For that we must turn to the Lewis model, which tells us how the electrons rearrange during the reaction. Information technology is as well of import to keep in mind why these reactions happen—they are caused by an electrostatic interaction between two oppositely charged parts of molecules: δ – is attracted to δ + .
One further notation, all reactions are initiated by random collisions of molecules, but only collisions that let the electrostatic interaction of the acid and base to occur are productive (that is, collisions that involve 2 similarly charged parts of molecules will not give rise to a reaction. Once again we volition have more to say near this afterward.
Acid–Base Reaction Direction and Position of Equilibrium
Acid base reactions begin because of electrostatic interactions, just the extent to which the reaction proceeds depends on the relative Gibbs gratuitous energy of the reactants and products, that is, the overall Gibbs free free energy change (ΔG) for the reaction. This is a subtle but important point: the reaction does non occur because the products are more than stable, it occurs considering there is an attractive force between 2 reactants that have polar structures, Equally nosotros volition come across, we can predict the relative amounts of reactants and products in a mixture (at equilibrium), based both on an understanding of molecular structures and by comparing their pKa.
Acid Strength (using the Brønsted–Lowry model): The forcefulness of an acid, that is the degree to which information technology donates H+ to (or accepts electron pairs from) other molecules, depends on a number of factors including, manifestly, the force of the base (that is the degree to which the base of operations donates electron pairs to other molecules) it reacts with. Acid and base strengths are usually reported using water as the solvent (i.east. as the base or acid respectively), so that acrid strengths tin be compared directly. Since biological reactions take place in aqueous solution we will be able to extend our understanding of simple acid base reactions to much more complex ones as we move frontwards.
The reaction for any acid HA is:
HA + H2O ⇄ H3O+ + A–
Nosotros can guess the extent of the reaction (i.eastward., how far the reaction goes, that is the concentrations of reactants and products when the reaction reaches equilibrium) by determining the equilibrium constant Ka.
Yarda = [H3O+][ A–]/[ HA]
In contrast to strong inorganic acids (such equally HCl, or HNO3), the equilibrium constants for many organic acids are modest (ranging from 10–1 to x-55) and it is more mutual to report pKa – which, as y'all will remember, is = –log K a . A stiff acid such as HCl has a large Ka (in fact it is so big equally to be meaningless) and therefore a very small (negative) pKa.
Some representative Ka and pKa values.
| Acid | Ka | pKa |
| HCl (muriatic acid) | ~107 | –7 |
| CFthreeCOOH (Trifluoroacetic acid) | 3.2 10 ten–ane | 0.5 |
| HF (hydrofluoric acid) | 7.2 x ten–4 | 3.14 |
| CH3COOH (acetic acid) | one.eight 10 ten–5 | 4.8 |
| Hii0 | 10-fourteen | fourteen |
| CHthreeCH2OH (acerb acid) | 10-16 | 16 |
| NHiv + (ammonia in NH4Cl) | 5.6 x ten–10 | 9.25 |
| CH4 (marsh gas) | ~10–55 | 55 |
It helps to be able to interpret these numbers in terms of the extent of the associated reaction. For instance, h2o (which acts as both an acid and a base) dissociates to a very small extent. In a liter of pure water, which contains ~54 moles of h2o molecules (or ~54 x 6.02 x 1023 molecules or ~3.25 ten x25 molecules), ~10-7 moles (or ~ten-7 x 54 x 6.02 10 1023 molecules or ~3.25 x ten16 H3O+ ions). The weaker the acid the higher the pKa (tin can you lot explain why that is the case and what it means in terms of the relative concentrations of species at equilibrium?).
It will assistance you profoundly if you memorize a few of import estimate pK a values for common acids, for example alcohols tend to have a pKa of ~15, while amines have a pKa ~33. Equally nosotros will see the pKa of diverse carbon species is very dependent on the environs of the C-H bond, but remembering that sp3 carbon-hydrogen bonds (pKa ~55) are non likely to ionize under any circumstances is helpful. Nonetheless, it is even more important to understand the factors that affect acrid force, and be able to apply them to predict and explain the outcomes of reactions.
Another important thought to think is that the extent of a reaction (as measured by its equilibrium constant G) is related to the change in Gibbs free energy (ΔG° = ΔH° – TΔS°) associated with that reaction. That is when we retrieve about the extent of a reaction (the concentration of reactants and products when the reaction reaches equilibrium) in terms of the relative stabilities of the reactants and products we need to take into account both the enthalpy modify (ΔH°), which reflects the changes in bonding and intermolecular interactions involving both reactants and products, and the entropy change (ΔS°) associated with the reaction system. Call up that ΔS° reflects change in the number of possible energy states and positions in the reaction system. For most organic (weak) acids, it turns out that the ΔH° of the dissociation reaction in h2o is approximately zero, because the types of bonding and interactions that are broken and formed during the reaction are like. Differences in ΔG for the reaction (and therefor Ka and pKa) are typically due to differences in ΔS.
- Explain why acids and bases are always (as pairs) constitute together in a system.
- What is meant by the terms conjugate acrid or conjugate base of operations?
- In the Lewis model for the HCl + water reaction, explicate why yous draw the arrow pointing from O to H.
- Complete these acid base reactions and predict the relative amounts of reactants and products when the reaction reaches equilibrium for each reaction. Explain your predictions using your knowledge of diminutive and molecular structures and electronegativity.
CHthreeNHii + HCL ⇄
CHthreeNHtwo + H20 ⇄
CH3NH– + H20 ⇄
CHiiiNHthree + + Htwo0 ⇄
Organic Acids and Bases
Having reviewed acids and bases using rather simple molecules (HCl and NH3), let us motility on to the more than complex world of organic acids and bases, how to place them, how to determine relative strengths, and how to predict what will happen in any given mixture. Nosotros brainstorm by comparing the pKa'due south of some organic acids. Permit us begin with ethanol (pKa ~sixteen), a molecule that we typically do non consider to be an acid, and acetic acid (pKa 4.8). At that place is clearly a huge difference between the pKa's of these two molecules, the question is can nosotros understand why this is the case?
If nosotros draw out their structures we meet that both have (as expected) the acidic hydrogen bonded to the electronegative oxygen. (Make sure yous call up why the hydrogens bonded to carbons are non as acidic as those bonded to oxygen). So why the huge divergence in pKa's? To answer this question we have to retrieve that the extent of the reaction depends on the relative thermodynamic stability of the products—that is, the system containing the cohabit base of the acid and the hydronium ion. The reactions and conjugate bases of the two are shown here (↓). 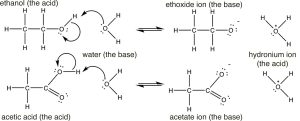 Based on their pKa values, nosotros would predict that the ethanol dissociation reaction is rare (few ethoxide ions form) while the acetic acrid dissociation reaction is more frequent. However note that even in the instance of the acetic acid merely about 3% of the acid molecules are dissociated in a 1M solution.
Based on their pKa values, nosotros would predict that the ethanol dissociation reaction is rare (few ethoxide ions form) while the acetic acrid dissociation reaction is more frequent. However note that even in the instance of the acetic acid merely about 3% of the acid molecules are dissociated in a 1M solution.
The first step in both reactions appears to exist more or less the same, an electron pair from the oxygen in h2o forms a bond to the electron scarce hydrogen while the O–H bail of the acrid breaks and the electrons originally associated with it the motility back to the oxygen. The deviation betwixt the two reactions lies mainly in the manner that the negatively charged cohabit bases (ethoxide and acetate) behave, and the way that they are solvated by the solvent (water). For ethoxide (ethanol's conjugate base), the actress negative accuse is localized onto the oxygen, which leads to a concentration of charge. H2o molecules are strongly attracted to the ethoxide anion, an interaction that limits the mobility of the Resonance Structures Resonance Hybrid water molecules and results in a decrease in entropy (ΔS is negative). In contrast, in acetate (acetic acid's conjugate base), the negative charge is delocalized onto both oxygens (even though it is often drawn every bit if it was associated with ane but not the other). We can illustrate this in two ways (or more than!) past drawing arrows to indicate how the extra electron pair can move from 1 oxygen to the other; it looks like this (→).
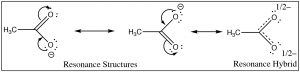 The actual structure has a partial negative charge on both oxygens. This pair of structures is frequently referred to as a resonance construction and the process is termed resonance but the proper name is misleading. In fact the actual structure, the resonance hybrid, does NOT involve the electrons (and the double bail) moving back and along between the ii oxygen atoms. By a biological (and not completely sensical) analogy we might say information technology is a mule or a hinny—the offspring of a cross between a horse and a donkey.[6] Merely equally a mule (or a hinny) is not bouncing dorsum and forth betwixt beingness a horse and beingness a donkey, so the resonance hybrid actually exists equally a new species[7], with an actual structure that is partway between the 2 (drawn) resonance structures. In this example, we are using two bonding models (a valence bond and a delocalized molecular orbital model) to describe the structure of acetate anion. The localized valence bond model involves a sigma single bond framework that connects the atoms and provides the molecular shape. The delocalized molecular orbital model describes a pi bond that connects both Os to the C. We can visualize the anion every bit a planar spii hybridized carbon connected to a methyl group and 2 oxygens past sigma bonds together with a iii cantlet ii electron pi bail that extends over the O–C–O framework (→). The outcome is that in the acetate ion the negative accuse is delocalized over ii oxygens, rather than existence concentrated on only i cantlet as it is in the
The actual structure has a partial negative charge on both oxygens. This pair of structures is frequently referred to as a resonance construction and the process is termed resonance but the proper name is misleading. In fact the actual structure, the resonance hybrid, does NOT involve the electrons (and the double bail) moving back and along between the ii oxygen atoms. By a biological (and not completely sensical) analogy we might say information technology is a mule or a hinny—the offspring of a cross between a horse and a donkey.[6] Merely equally a mule (or a hinny) is not bouncing dorsum and forth betwixt beingness a horse and beingness a donkey, so the resonance hybrid actually exists equally a new species[7], with an actual structure that is partway between the 2 (drawn) resonance structures. In this example, we are using two bonding models (a valence bond and a delocalized molecular orbital model) to describe the structure of acetate anion. The localized valence bond model involves a sigma single bond framework that connects the atoms and provides the molecular shape. The delocalized molecular orbital model describes a pi bond that connects both Os to the C. We can visualize the anion every bit a planar spii hybridized carbon connected to a methyl group and 2 oxygens past sigma bonds together with a iii cantlet ii electron pi bail that extends over the O–C–O framework (→). The outcome is that in the acetate ion the negative accuse is delocalized over ii oxygens, rather than existence concentrated on only i cantlet as it is in the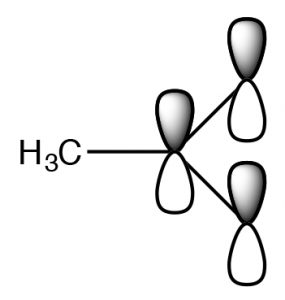 ethoxide ion. The result is that the interactions of the acetate with solvent water molecules is not as strong, and then that the h2o molecules are not as ordered, meaning that the water is not as ordered around the molecule and the entropy change is non as negative. The effects of delocalizing charge over more than 1 atom play a major role in predicting the outcomes of a wide range of reactions. We note that ΔS is yet negative since the creation of a charged species however leads to increased ordering of solvent molecules.
ethoxide ion. The result is that the interactions of the acetate with solvent water molecules is not as strong, and then that the h2o molecules are not as ordered, meaning that the water is not as ordered around the molecule and the entropy change is non as negative. The effects of delocalizing charge over more than 1 atom play a major role in predicting the outcomes of a wide range of reactions. We note that ΔS is yet negative since the creation of a charged species however leads to increased ordering of solvent molecules.
One mode to predict whether charge tin can be delocalized is to make up one's mind whether resonance structures can exist drawn for the charged species. For instance: try convincing yourself that y'all cannot draw resonance structures for ethanol.
Resonance is not the only way to stabilize accuse. Typically, resonance occurs through aconjugated pi bond system, such as occurs inside the –CO2 – role of an organic acid, but how do we account for the departure in the acidities of acerb acid (pKa 4.viii) and trifluoroacetic acid (→) (pKa 0.5), even though they both have the carboxylate functional grouping? The divergence between the ii lies in the fact that the accuse on the trifluoroacetate anion is delocalized by ii distinct mechanisms. Equally in acetate, the negative charge is delocalized past resonance 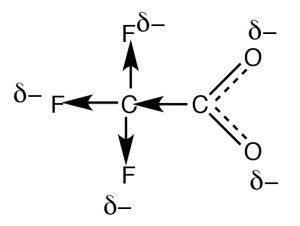 through the pi bonding system; in improver it is too delocalized onto the fluorines by the fact that the highly electronegative fluorine atoms (more electronegative than O) withdraw electrons from the methyl carbon through the sigma bonds, which in turn withdraws electrons from the adjacent carbon, and in turn from the two oxygens (a procedure known as "consecration"). The result is that the negative charge is "smeared out" over fifty-fifty more atoms, making the anion even less likely to cause a solvent molecule ordering (reducing the effect on ΔS). As you might expect, the inductive issue is altitude dependent (perchance you can predict the outcome of calculation more CH2 groups between the CF3 and COtwo groups).
through the pi bonding system; in improver it is too delocalized onto the fluorines by the fact that the highly electronegative fluorine atoms (more electronegative than O) withdraw electrons from the methyl carbon through the sigma bonds, which in turn withdraws electrons from the adjacent carbon, and in turn from the two oxygens (a procedure known as "consecration"). The result is that the negative charge is "smeared out" over fifty-fifty more atoms, making the anion even less likely to cause a solvent molecule ordering (reducing the effect on ΔS). As you might expect, the inductive issue is altitude dependent (perchance you can predict the outcome of calculation more CH2 groups between the CF3 and COtwo groups).
- Using resonance structures predict which is more than acidic: C6H5OH or CH3CH2OH?
- Draw structures to show how sodium ethoxide and sodium acetate are solvated in water, and use them to evidence why the negative entropy change for the formation of sodium acetate is smaller than that of sodium ethoxide.
- Consider the pKa's of the three chlorobutanoic acids: CHiiiCHiiCHClCOOH (pKa ii.86), CHiiiCHClCH2COOH (pKa four.05), and CH2ClCH2CH2COOH (pKa 4.53). Draw structures and utilize them to explicate why these carboxylic acids have unlike pKa'southward.
Organic Bases
As noted previously, there are no acids without bases, and vice versa. Fifty-fifty if we are only discussing H+ (proton) transfer, it is (arguably) easier to think near the base using a Lewis model. That is, a base has an electron pair available for donation into a bond with the acid. Think that almost everything that has a pair of non-bonding electrons (sometimes called a lone pair) tin can act every bit a base of operations. The well-nigh mutual types of organic bases often have a nitrogen atom somewhere in their structure. If we compare the basicity of Due north, O and F, each of which have lone pairs that are could potentially be donated, nitrogen is the least electronegative and therefore the best able to donate its electrons into a bail, since its lonely pair is least attracted by the nucleus. Fluorine, the most electronegative element, holds its electrons very close to the nucleus, and under normal circumstances would non be considered equally a base.
Oxygen, since it is more electronegative than nitrogen is not as strong a base of operations, therefore when ammonia and h2o are mixed, the simply reaction that occurs (and that to a relatively small extent) is a proton transfer from h2o to ammonia.
NHthree + H2O ⇆ NH4 + + –OH
The equilibrium constant for this reaction is 1.viii ten 10–v (most of the species in the mixture at equilibrium are reactants)
*insert image hither*
Hither are some organic bases (→). Annotation that they are components of a wide range of biologically agile molecules, including Deoxyribonucleic acid, hormones and pharmaceuticals. Equally we volition see the basic nitrogen provides an important manner to sympathise the reactivity of a item species.
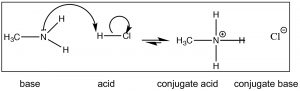 For at present, however, let us first with a simpler base such as methylamine (CH3NHii) the simplest nitrogenous organic base. Methylamine reacts with acids (↓) in much the same fashion that ammonia does; it will react with a stiff acid like HCl(aq) to produce methylammonium chloride.
For at present, however, let us first with a simpler base such as methylamine (CH3NHii) the simplest nitrogenous organic base. Methylamine reacts with acids (↓) in much the same fashion that ammonia does; it will react with a stiff acid like HCl(aq) to produce methylammonium chloride.
Call up that the position of equilibrium can be predicted by comparing the force (pKa's) of the two acids. HCl (pKa –7) is a much stronger acid than CH3NHiii + (pKa ~10) and therefore we predict that the equilibrium of the methylamine + HCl reaction will lie well to the right. Now consider the reaction in which methylamine reacts with acetic acid (↓).
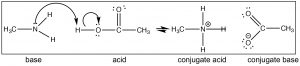
Over again we can predict the position of equilibrium by comparison pKa's of the conjugate acids (acetic acrid 4.viii and CHiiiNH3 + ~ 10). Discover that y'all tin can predict the construction of the products simply by following the flow of electrons. We could alter the CH3 (methyl) groups on either methylamine and acerb acid to a wide range of unlike groups and still be able to predict the product hands, as long every bit you lot recognize that the reaction that takes place is a (simple) proton transfer (acid–base). For case, wait at the structure of cocaine (higher up): can you lot predict what volition happen if it were reacted with acetic acid? What would be the structure of the production?
Molecules that contain both an acid and a base of operations:
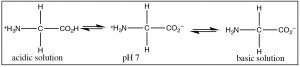
The nearly common example of a molecule that deed as both an acid and a base is of course water because it has both a potentially acidic hydroged, and a lone pair that can accept the proton. Nevertheless, since this is organic chemistry, where water is not equally common a solvent, allow us consider the class of molecules that have both acidic and basic domains simultaneously. The well-nigh biologically important such molecules are the amino acids, which take both an amino grouping and a carboxylic acid. A subset of the possible amino acids are those used in biological systems to get together polypeptides. Amino acids (or rather the α-amino acids) contain both a carboxylic acid and an amino group fastened to a central carbon (the α-carbon). The generic structure is given hither (→) where R stands for a wide range of side bondage.[8] 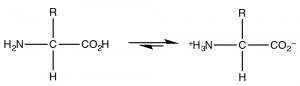 At pH 7 the amino acrid exists in what is know as a zwitterionic class, in which the carboxylic acid grouping is negatively charged while the amino grouping is positively charged. At no fourth dimension would an amino acid (dissolved in water) exist in an un-ionized grade. We can predict what class would be present at different pH's past considering the pKa'due south of the species involved.
At pH 7 the amino acrid exists in what is know as a zwitterionic class, in which the carboxylic acid grouping is negatively charged while the amino grouping is positively charged. At no fourth dimension would an amino acid (dissolved in water) exist in an un-ionized grade. We can predict what class would be present at different pH's past considering the pKa'due south of the species involved.
Effect of pH on acid base reactions
And then far, nosotros have discussed situations when the acid or base of operations is added to solution of pure water. Pure water has a pH of seven, and [H3O+] = [–OH]. Now allow u.s. consider what happens when nosotros change the pH of the solution. For example consider a state of affairs in which nosotros dissolve a simple organic acrid (CH3CO2H, acetic acid) in a solution that has a pH > 7, that is where the [–OH] > [H3O+]; under these conditions the extent of the acetic acid's ionization is increased. 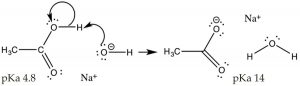 Call back that in 1M acetic acrid just ~three% of the acid is ionized at pH 7. If we modify the solution to make information technology basic by calculation NaOH, the excess of strong base (–OH) will completely deprotonate the acid. At equilibrium, the reaction will now favor products over reactants (i.e. it volition motion to the right). What we accept done here is drive the acetic acid ⇆ acetate reaction to the right, increasing the concentration of acetate, which is an awarding of Le Châtelier's principle). Note that Na+, derived from the addition of the NaOH used to adapt the pH, is nowadays but does not accept part in the reaction – for this reason it referred to equally a "spectator ion". Another, perhaps simpler, style to predict the consequence of this reaction is to use the pKa values of the 2 acids (CH3CO2H, iv.8 and H2O, 14), conspicuously acetic acrid is a much stronger acrid than h2o, and therefore the equilibrium position for this reaction will lie over to the right in favor of the weakest acid and the weakest base. What we have done here is change the acetic acrid, which is a polar organic molecule, into acetate, an ionic species.
Call back that in 1M acetic acrid just ~three% of the acid is ionized at pH 7. If we modify the solution to make information technology basic by calculation NaOH, the excess of strong base (–OH) will completely deprotonate the acid. At equilibrium, the reaction will now favor products over reactants (i.e. it volition motion to the right). What we accept done here is drive the acetic acid ⇆ acetate reaction to the right, increasing the concentration of acetate, which is an awarding of Le Châtelier's principle). Note that Na+, derived from the addition of the NaOH used to adapt the pH, is nowadays but does not accept part in the reaction – for this reason it referred to equally a "spectator ion". Another, perhaps simpler, style to predict the consequence of this reaction is to use the pKa values of the 2 acids (CH3CO2H, iv.8 and H2O, 14), conspicuously acetic acrid is a much stronger acrid than h2o, and therefore the equilibrium position for this reaction will lie over to the right in favor of the weakest acid and the weakest base. What we have done here is change the acetic acrid, which is a polar organic molecule, into acetate, an ionic species.
Acetic acid is a pocket-size organic molecule; since information technology is polar it can collaborate with h2o (though intermolecular forces), therefore acetic acrid is very soluble in water (indeed it is miscible with water (it has unlimited solubility.)[nine] But now, let usa consider the effect of increasing the length of the hydrocarbon group of the organic acid on its molecular properties. Acetic acid has a methyl (CH3–) group, the smallest possible hydrocarbon. In dissimilarity dodecanoic (lauric) acid has a 12-carbon hydrocarbon concatenation (CHiii[CH2]eleven–) and has a solubility in water of 0.063 g/L (~30 mM) at 25 °C, which is much less that of acetic acrid.[10] As the hydrocarbon (non-polar) part of the molecule increases in length, solubility in h2o decreases: the ΔG of the procedure of dissolving the organic acrid in h2o becomes more positive. This decrease in solubility is primarily due to a negative entropy change (ΔS) acquired by the self-organization of water molecules around the hydrocarbon "tail" of the molecule.  Now let u.s. consider the behavior of ionized sodium dodecanoate (the sodium table salt of dodecanoic acid); it, like many ionic species, it is soluble in water. Although every bit y'all may recall, this is a different form solubility – the soluble species is not isolated molecules simply rather molecular complexes known as micelles (→).[xi] The upshot of this is nosotros tin can "solubilize" organic acids in h2o past deprotonating them, but if we and then lower the pH, the organic acid will split from solution once again.
Now let u.s. consider the behavior of ionized sodium dodecanoate (the sodium table salt of dodecanoic acid); it, like many ionic species, it is soluble in water. Although every bit y'all may recall, this is a different form solubility – the soluble species is not isolated molecules simply rather molecular complexes known as micelles (→).[xi] The upshot of this is nosotros tin can "solubilize" organic acids in h2o past deprotonating them, but if we and then lower the pH, the organic acid will split from solution once again.
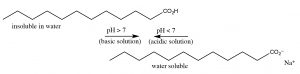
Organic bases can be solubilized in a similar way, except that now the solution must be made acidic. For example, a nitrogenous base with a large non-polar group such as dodecylamine (C12H27Northward) has a solubility of near 3.five g/L (~20mM), merely at acidic pHs it is completely soluble. Contrast the solubility of dodecyl amine with cadaverine (NH2CH2CH2CH2CH2CHtwoNHii), the compound that smells like its name, which is completely miscible with water because information technology has two polar amino groups. It turns out that nosotros can predict the pH at which a detail acid or base of operations protonates or deprotonates. Y'all may remember from full general chemistry that the pH of weak acids and their conjugate bases (like most organic species) can be described using the Henderson Hasselbalch equation (→).
1 way to piece of work with this equation is to note that [acid] = [conjugatepH =pKa + log [latex]\frac{[base]}{[acid]}[/latex] base] when the pH of the system is equal to pKa of the acrid. At pH's below pKa, [acid] > [conjugate base], in that location is more than acrid than base of operations, and vice versa for pH > pKa. Therefore, by adjusting the pH nosotros can change the concentrations of conjugate acid and base to arrange our purposes, or we tin can predict the relative concentrations at whatsoever pH. For case, acetic acrid with a pKa of four.eight would accept l% CHthreeCO2H, and 50% CHthreeCOtwo –Na+, at a pH of 4.eight. If the pH falls below 4.8 the concentration of protonated acid volition increase, and if information technology rises the concentration of acetate ion will increase.
This ability to transform an organic substance from an insoluble (in water) molecule to a soluble ionic species can be very useful. Ane mutual case stems from the fact that many pharmaceutical drugs are organic substances that are insoluble in aqueous solutions (like cytoplasm or blood). If these substances were introduced into the trunk in their not-ionized form they would not dissolve, and therefore be inactive. If you lot cheque the labels on many prescription bottles you lot will see that the drug is administered as a salt. Consider norepinephrine (→), a hormone that is oftentimes administered intravenously to counteract the effects of allergic reactions. 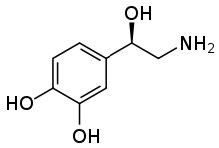 It is administered as a salt of tartaric acid to ensure that it is soluble in the claret stream.
It is administered as a salt of tartaric acid to ensure that it is soluble in the claret stream.
You may come across another example of this phenomenon (that acids are soluble in bones solution, and bases are soluble in acid solution) if you take the organic chemistry laboratory grade. If your product has an acidic or basic moiety in its structure, you can extract the substance into aqueous (acrid or basic) solution, washing away all the organic by-products with an organic solvent, and and then regenerating the acidic or basic substance. This is an important purification method for many substances, because it allows the compound of interest to be separated into aqueous solution and and so regenerated but by adding or subtracting a proton.
 When nosotros consider biomolecules (that is, organic molecules found in organisms) the situation is not then clear cutting; most biomolecules have a variety of acidic and basic groups every bit role of their structure. Even the simplest amino acid, glycine (→) exist in a variety of protonated and deprotonated forms depending on the pH.
When nosotros consider biomolecules (that is, organic molecules found in organisms) the situation is not then clear cutting; most biomolecules have a variety of acidic and basic groups every bit role of their structure. Even the simplest amino acid, glycine (→) exist in a variety of protonated and deprotonated forms depending on the pH.
I thing that becomes clear is that individual amino acids are always charged regardless of the pH, so they are water-soluble. Only the extent of the protonation/deprotonation reactions is pH dependent. Equally nosotros will see this has a number of ramifications for a broad range of biological molecules, because they will comport very differently in different pH solutions. This is one important reason why near biological systems are buffered so that they remain at a fairly abiding pH.
- If yous have a mixture of benzoic acid Chalf-dozenHvCO2H (pKa four.two), toluene, C6H5CHthree and aniline hydrochloride (pKa of CsixH5NH3 + 4.half-dozen). Which substance will exist soluble in aqueous acidic solution, which will exist soluble in aqueous basic solution, which will non be soluble in water?
- Outline a scheme for separating these three substances by using their differing solubilities in organic and aqueous solutions of different pHs.
Lewis Acids and Bases, Electrophiles and Nucleophiles
As we accept seen, any reaction in which a proton (H+) is transferred from one molecule to some other can be considered as a Lewis acid–base of operations reaction, but now it is time to augment the telescopic of Lewis acid–base reactions. The structural requirement for a Lewis base is substantially the same as those we discussed for a Brønsted base. That is, a Lewis base must accept an accessible lone pair of electrons that can be donated into a bond with a Lewis acid. 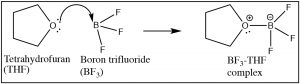 For example, many (but not all) nitrogen and oxygen containing molecules have such available alone electron pairs and so tin be considered every bit Lewis bases.[12] Information technology is the Lewis acid that tin can have a number of different forms (and and so, tin be harder to recognize). A Lewis acrid must exist able to take a pair of electrons. In practice this means a multifariousness of substances (besides H+) can act as Lewis acids: for case, any species with empty orbitals that are energetically accessible can exist a Lewis acid.
For example, many (but not all) nitrogen and oxygen containing molecules have such available alone electron pairs and so tin be considered every bit Lewis bases.[12] Information technology is the Lewis acid that tin can have a number of different forms (and and so, tin be harder to recognize). A Lewis acrid must exist able to take a pair of electrons. In practice this means a multifariousness of substances (besides H+) can act as Lewis acids: for case, any species with empty orbitals that are energetically accessible can exist a Lewis acid. 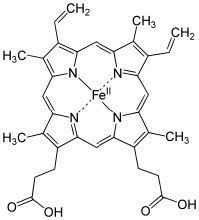 Common examples of this situation are compounds of Group 3 elements (specifically B and Al); these have only iii valence electrons. Examples include BF3 (↑) and AlCl3,[13] both of which have a fractional positive charge on the cardinal atom and an empty orbital that can accept electrons. Other examples of Lewis acids are metal ions such every bit Fe2+, Atomic number 263+, Cu2+, and Mgtwo+; these, by definition, have empty orbitals. The aforementioned situation holds true for many transition metal salts, for example TiCl4 and NiCl2.[14] In biological systems, examples of Lewis acid–base complexes include the active site of the oxygen send complex in hemoglobin (and myoglobin), which consists of an iron ion complexed with 4 nitrogens, which are role of a porphyrin ring. A similar iron-porphyrin complex is institute associated with the cytochrome proteins that participate in the ATP synthesis reaction associated with in the electron ship concatenation of the mitochondria (→). Chlorophyll, the light-green pigment that is part of the low-cal capture arrangement in algae and plants has a similar structure, except that the Lewis acid at the centre of the circuitous is Mg2+ rather than Fe2+. This has the interesting effect of making chlorophyll species appear to be dark-green, rather than the carmine observed in blood. This is caused by the difference free energy gaps between the molecular orbitals in an Iron complex as compared to a Mg complex with a porphyrin band. We will discuss this effect in more detail later. As we will also meet later, Lewis acids are important class of reagents in organic chemistry because they tin collaborate with a wide range of bases.
Common examples of this situation are compounds of Group 3 elements (specifically B and Al); these have only iii valence electrons. Examples include BF3 (↑) and AlCl3,[13] both of which have a fractional positive charge on the cardinal atom and an empty orbital that can accept electrons. Other examples of Lewis acids are metal ions such every bit Fe2+, Atomic number 263+, Cu2+, and Mgtwo+; these, by definition, have empty orbitals. The aforementioned situation holds true for many transition metal salts, for example TiCl4 and NiCl2.[14] In biological systems, examples of Lewis acid–base complexes include the active site of the oxygen send complex in hemoglobin (and myoglobin), which consists of an iron ion complexed with 4 nitrogens, which are role of a porphyrin ring. A similar iron-porphyrin complex is institute associated with the cytochrome proteins that participate in the ATP synthesis reaction associated with in the electron ship concatenation of the mitochondria (→). Chlorophyll, the light-green pigment that is part of the low-cal capture arrangement in algae and plants has a similar structure, except that the Lewis acid at the centre of the circuitous is Mg2+ rather than Fe2+. This has the interesting effect of making chlorophyll species appear to be dark-green, rather than the carmine observed in blood. This is caused by the difference free energy gaps between the molecular orbitals in an Iron complex as compared to a Mg complex with a porphyrin band. We will discuss this effect in more detail later. As we will also meet later, Lewis acids are important class of reagents in organic chemistry because they tin collaborate with a wide range of bases.
Electrophiles and Nucleophiles
The side by side logical stride in expanding our ideas about Lewis acids and bases is to consider reactions that involve carbon. We will get-go consider reactions in which carbon acts like the Lewis acid, that is, it accepts a pair of electrons to course a new bond with a Lewis base of operations. Then, what situations would we make a carbon human action in this manner? We can dominion out (for now) carbon compounds with an empty orbital (akin to boron). Why? Because all stable carbon compounds grade 4 bonds and there are no depression-lying empty orbitals that tin be used to take electrons.
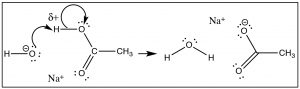 But let united states of america first await at the proton (H+) transfer reaction as a model (→). In this case the bond with the Lewis base (OH-) is formed at the same time as the bail to the conjugate base (of the acid) is cleaved. We see that the δ+ on the H means that the bond to the H is partially ionized. The H is "on the fashion" to condign H+—a species that does have an empty and accessible orbital. The δ+ on the H attracts the negative (or δ–) charge on the base, and the reaction is initiated, forming a new bond betwixt the O and the H, and at the same time breaking the old O–H bond.
But let united states of america first await at the proton (H+) transfer reaction as a model (→). In this case the bond with the Lewis base (OH-) is formed at the same time as the bail to the conjugate base (of the acid) is cleaved. We see that the δ+ on the H means that the bond to the H is partially ionized. The H is "on the fashion" to condign H+—a species that does have an empty and accessible orbital. The δ+ on the H attracts the negative (or δ–) charge on the base, and the reaction is initiated, forming a new bond betwixt the O and the H, and at the same time breaking the old O–H bond.
Nosotros can imagine that a carbon chemical compound with a δ+ on the C might behave in a very like manner. 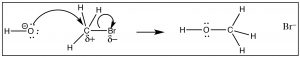 In this molecule (HthreeCBr) the C-Br bond is polarized so that at that place is a small positive charge on the C, which attracts the negatively charged hydroxide (→). Germination of the O–C bail occurs with the simultaneous breaking of the C-Br bail.
In this molecule (HthreeCBr) the C-Br bond is polarized so that at that place is a small positive charge on the C, which attracts the negatively charged hydroxide (→). Germination of the O–C bail occurs with the simultaneous breaking of the C-Br bail.
Consider the analogies between these 2 reactions – the mechanisms of how and why the electrons move are similar. The only real difference between the two reactions is that in the beginning an H+ is transferred from an O (on the carboxylic acrid) to the OH–, while in the 2nd, a methyl group is transferred to the OH–. Now for a change in nomenclature: when such a reaction involves a C atom (a carbon eye) rather than call the electron scarce carbon a Lewis acid, we telephone call it an electrophile (electron or negative charge loving). Similarly, the hydroxide ion (which acts as a Lewis base) is now called a nucleophile (positive accuse loving). This modify in terminology is not just to confuse students! In fact, there are subtle differences betwixt Lewis acids and bases and electrophiles and nucleophiles that brand the distinction between the two useful. In particular, while all Lewis bases are nucleophiles, every bit nosotros volition see, not all nucleophiles are bases.
So now we have to inquire ourselves, what factors brand a particular C within a molecule an electrophile? How tin we recognize a nucleophile? What criteria do nosotros utilize to gauge the forcefulness of a particular electrophile or an nucleophile? Tin we always go carbon to human action as a nucleophile? If nosotros can respond these questions, we can predict the issue of a wide array of reactions.
What makes a particular carbon an electrophile?
The simplest of organic compounds are hydrocarbons, and the simplest of hydrocarbons are known equally alkanes. Alkanes typically have the formula CnH2n+2 (or if Cdue northH2n if there is one ring of carbons, subtract 2H for every extra ring). All of the bonds within an paraffin are sigma (single) bonds; they do not contain pi (double) bonds.[15] In an alkane, each carbon is fully saturated, it makes four unmarried bonds and (as noted to a higher place) there are no double or triple bonds. C-C bonds are of form, completely not-polar since the electrons are as distributed between ii identical atoms, however C-H bonds are also relatively non-polar since the electronegativities of C and H are quite similar. In do this means that alkanes are express in their reactivity. The most common reactions that an alkane tin can take part in are reactions with oxygen to produce CO2 and HtwoO. This reaction is highly exothermic, although there is a significant activation energy, so it requires an initial input of free energy (typically a spark, a burning friction match) to start the reaction, merely so the free energy from the formation of the potent C=O and O-H bonds (which is why the reaction is exothermic) can be used to initiate more than reaction. The actual reaction mechanism is circuitous; it proceeds via a serial of highly reactive (unstable) free radicals (species with unpaired electrons)[sixteen]. While this reaction is obviously highly important—this is still how we generate much of the energy to run our cars and electrical power stations, from an organic chemistry perspective it is not very interesting in large office considering information technology is more than or less uncontrollable. That is, if you have enough oxygen one time started the reaction generates COii and H2O, regardless of which hydrocarbon you begin with. [17]
All this is to say that alkanes are not good candidates for the kinds of reactions we are considering, they have neither nucleophile nor electrophilic carbons. And then, let u.s. turn our attention to carbon compounds with elements other than C and H and both sigma and pi bonds (this is, of course, the rest of organic chemistry). Here nosotros find a very different situation: the range of reactions and the types of products tin seem nearly unlimited. While information technology is impossible (and certainly undesirable) to memorize every reaction and every potential production, it is possible to organize your understanding of chemical systems then that you can brand plausible predictions as to which reactions may occur. Past knowing reaction mechanisms, and when they are relevant, you lot tin can also predict which reactions volition occur and therefore what products volition form. Equally y'all might recognize, this is the aforementioned strategy nosotros have used to consider acrid–base reactions, which can be understood much more broadly than uncomplicated proton (H+) transfer reactions. Thinking in an electrophile-nucleophile context provides an entrée into much of organic chemical science.
For reactions (other than reactions involving free radicals, like combustion) to occur, in that location is more often than not a "handle" within the substrate: a place where the electron density is not evenly distributed, a site at which reactants of opposite charge collaborate (and react). In the example we used previously, the electrophilic carbon has a δ+ on it; this fractional charge arose because the C was bonded to a more electronegative element. Such a partially positively charged C is attractive to whatsoever species with a negative (or partial negative) charge. 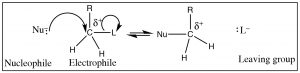 Note that, for now, we are going to restrict the blazon of carbon atom that nosotros are considering to either a primary (that is a carbon with just one alkyl group (denoted by R) and 2 hydrogens, CH2R–) or a methyl carbon (CHiii–). As nosotros volition see things go more than complicated when we starting time to add together more alkyl groups around the site of set on—so nosotros will come dorsum to that afterward.
Note that, for now, we are going to restrict the blazon of carbon atom that nosotros are considering to either a primary (that is a carbon with just one alkyl group (denoted by R) and 2 hydrogens, CH2R–) or a methyl carbon (CHiii–). As nosotros volition see things go more than complicated when we starting time to add together more alkyl groups around the site of set on—so nosotros will come dorsum to that afterward.
To identify such a partially positively charged C 1 would look for C's bonded to groups (atoms) that are more electronegative, that is, that will act to withdraw electrons from the carbon (denoted by L below). But since carbon cannot form more than than iv bonds as the nucleophile comes in and forms a bond, another bond must pause. The electronegative atom (L) (or group of atoms), is known as the "leaving group" (oh, how dull) needs to be stable when it leaves with the extra pair of electrons. We can, in fact, predict the characteristics of a proficient leaving group. For example, the bond to the leaving group should exist polarized, and since the leaving group takes the electron pair with it, the grouping should exist stable with this actress pair of electrons on it (Fifty–). Another way of saying this is that the leaving group should be electronegative and breaking the C-L bond should produce a weak base. Halide ions are examples of skilful leaving groups, and their lodge of reactivity is I– > Br– > Cl– > F–. This ranking mirrors their acid strength rankings—that is, Howdy is the strongest acid and HF is the weakest—which ways F– is the strongest base (and therefore to the lowest degree probable to leave)
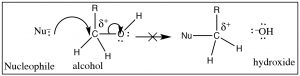 So, what about oxygen, in the form of an alcohol O–H grouping, as a leaving group? (→) Information technology certainly fulfills the requirement that the C–O bond be polarized, but if you follow the reaction through information technology would mean that the leaving group would be a hydroxide ion (– OH), a very strong base. Therefore, alcohols (ROH) are not likely to be attacked by a nucleophile.
So, what about oxygen, in the form of an alcohol O–H grouping, as a leaving group? (→) Information technology certainly fulfills the requirement that the C–O bond be polarized, but if you follow the reaction through information technology would mean that the leaving group would be a hydroxide ion (– OH), a very strong base. Therefore, alcohols (ROH) are not likely to be attacked by a nucleophile.
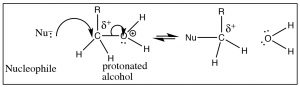 At that place are means, however, means to make an booze reactive. For example, if nosotros can carry out the reaction in an acidic solution, the alcohol will exist protonated (at least some of the time), and therefore the leaving group will be a water molecule, a stable entity (→).
At that place are means, however, means to make an booze reactive. For example, if nosotros can carry out the reaction in an acidic solution, the alcohol will exist protonated (at least some of the time), and therefore the leaving group will be a water molecule, a stable entity (→).
What makes a good nucleophile?
As we take noted, a Lewis base is also a nucleophile, so the trends you have learned virtually the strengths of Lewis bases also hold for nucleophiles. So, for case, nucleophilicity decreases beyond a row in the periodic table so NH3 > HtwoO > HF in the same manner as base of operations strength does (recall this is considering the solitary pair is more than bachelor on the N than on F). But since this is organic chemistry, we should have some organic groups dangling off the nucleophiles. 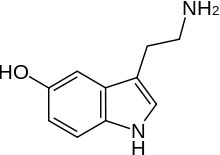 Then for instance, instead of a hydroxide nucleophile, we could use an alkoxide nucleophile (for example, CH3CH2O– Na+ sodium ethoxide), or we could apply amine nucleophiles like serotonin (the nitrogen in the NH2 group here is more nucleophilic than the OH group, and the N in the ring). In improver, if nosotros compare nucleophiles with the same nucleophilic atom, a negatively charged species is more nucleophilic than the uncharged form, so OH– > HiiO, and NHtwo– > NH3 (and past analogy whatsoever organic derivatives conduct the same way).
Then for instance, instead of a hydroxide nucleophile, we could use an alkoxide nucleophile (for example, CH3CH2O– Na+ sodium ethoxide), or we could apply amine nucleophiles like serotonin (the nitrogen in the NH2 group here is more nucleophilic than the OH group, and the N in the ring). In improver, if nosotros compare nucleophiles with the same nucleophilic atom, a negatively charged species is more nucleophilic than the uncharged form, so OH– > HiiO, and NHtwo– > NH3 (and past analogy whatsoever organic derivatives conduct the same way).
Too the nucleophiles that are easily recognizable considering they are bases, in that location is another grade of nucleophiles that are somewhat unlike; they have a lone pair of electrons, but they are not particularly basic. The most common examples are the halide ions, which are weak bases and good leaving groups. So, the question arises: why are halide ions such good nucleophiles? The reason for this has to exercise with their polarizability (that is, the extent to which an electron deject tin get distorted) of the nucleophile. A very large anion-like iodide has a very polarizable electron deject because the electrons extend much further out from the nucleus than, for instance, the electron deject in fluoride. This means that the electron cloud for iodide tin begin partial bail formation to the carbon much before than the one for fluoride, and therefore iodide reacts much faster than fluoride.[18] This logic allows united states to explain why the nucleophilicity of halide ions increases as you go down a group: I– > Br– > Cl– > F–.
Although we will return to this reaction in greater detail later, let u.s. accept a expect at the range of possible reactions that this generic scheme enables usa to predict – with the caveat that we are considering elementary carbon substrates. Reactions like this are called nucleophilic substitutions, because the species that attacks the carbon is a nucleophile, and the overall effect of the reaction is that we have substituted the nucleophile for the leaving grouping. This detail case is called an Due south N ii reaction which stands for Substitution, Nucleophilic, two nd Order, and we will come back to discuss the reaction in much more particular later.
Another blazon of carbon nucleophile
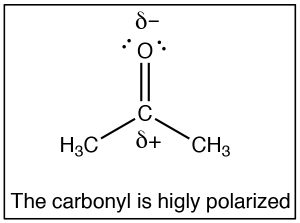 The SN2 reaction is a mainstay of organic chemistry, by varying the substrate (carbon electrophile) the leaving group, and the nucleophile we tin construct a huge assortment of unlike compounds. Another very of import type of compound that has an electrophilic carbon (i.e. a carbon that is subject to nucleophilic attack) is one which contains a carbonyl group (C=O). The carbonyl grouping is highly polarized, with a large δ+ on the carbon. This can exist rationalized by the idea that there are two bonds to the electronegative oxygen and therefore the oxygen has even more tendency to pull electrons abroad from the carbon than a single bonded oxygen would.
The SN2 reaction is a mainstay of organic chemistry, by varying the substrate (carbon electrophile) the leaving group, and the nucleophile we tin construct a huge assortment of unlike compounds. Another very of import type of compound that has an electrophilic carbon (i.e. a carbon that is subject to nucleophilic attack) is one which contains a carbonyl group (C=O). The carbonyl grouping is highly polarized, with a large δ+ on the carbon. This can exist rationalized by the idea that there are two bonds to the electronegative oxygen and therefore the oxygen has even more tendency to pull electrons abroad from the carbon than a single bonded oxygen would. 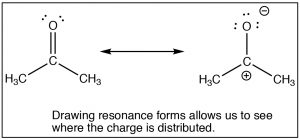 One way to visualize this is to draw resonance structures for the carbonyl grouping as shown, where the electrons from the double bond are now located on the O. We will come dorsum to how to draw resonance forms in much more detail later.
One way to visualize this is to draw resonance structures for the carbonyl grouping as shown, where the electrons from the double bond are now located on the O. We will come dorsum to how to draw resonance forms in much more detail later.
Once we understand how compounds with carbonyl groups are polarized, nosotros can predict (at to the lowest degree for the kickoff step) how these compounds will react. For example, if we have a reasonably skillful nucleophile (here shown as Nu–)we might predict that information technology would attack at the carbonyl carbon. 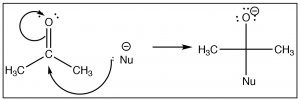 The difference in this reaction and an Due southNii reaction is that at that place is no leaving group. Instead the electrons from one of the C-O bonds move onto the oxygen as shown.
The difference in this reaction and an Due southNii reaction is that at that place is no leaving group. Instead the electrons from one of the C-O bonds move onto the oxygen as shown.
In that location are a number of ways that this reaction can keep, the most obvious of which is that if the reaction is in contact with a solvent that has acidic protons (e.g. water or an booze), the O– tin can merely protonate in an acid base reaction. As we will see later, the course of the reaction too depends on what the nucleophile is. Hither we volition requite the simplest example which is the reaction of a ketone (acetone) with a carbon nucleophile (CH3CH2Li, ethyl lithium). For at present, nosotros will not worry virtually how to brand ethyl lithium, just balance assured information technology is possible! When the negatively charged carbon electrophile adds to the carbonyl we make a new carbon-carbon bond. This is followed by addition of water to protonate the oxygen, to produce an booze. The overall reaction is a nucleophilic addition.
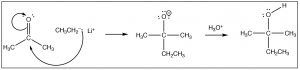
Try your hand at predicting the outcomes for these reactions by drawing arrow pushing mechanisms.
- CH3CH2I + NaOH →
- CH3Br + NaN3 →
- CH3CH2Cl + NHtwoCH3 →
- CH3OH + H+ →
What nucleophile and electrophile would yous react together to form these products?
- CH3OH + Br–
- CHthreeCH3NH3 + + Cl–
- Construct a generalizable model for the SNtwo reaction and explain the part of the substrate (the carbon electrophile), the leaving grouping, and the nucleophile.
Construct a generalizable model for the nucleophilic improver reaction and explain the role of the substrate (the carbon electrophile), and the nucleophile. What functional groups would undergo a nucleophilic addition? - What would make a carbon in a compound a nucleophile? How could you go about making a detail carbon nucleophilic?
Complete The Following Acid-base Reaction.,
Source: https://openbooks.lib.msu.edu/oclue/chapter/chapter-1-acid-base-reactions/
Posted by: morristwounds.blogspot.com


0 Response to "Complete The Following Acid-base Reaction."
Post a Comment